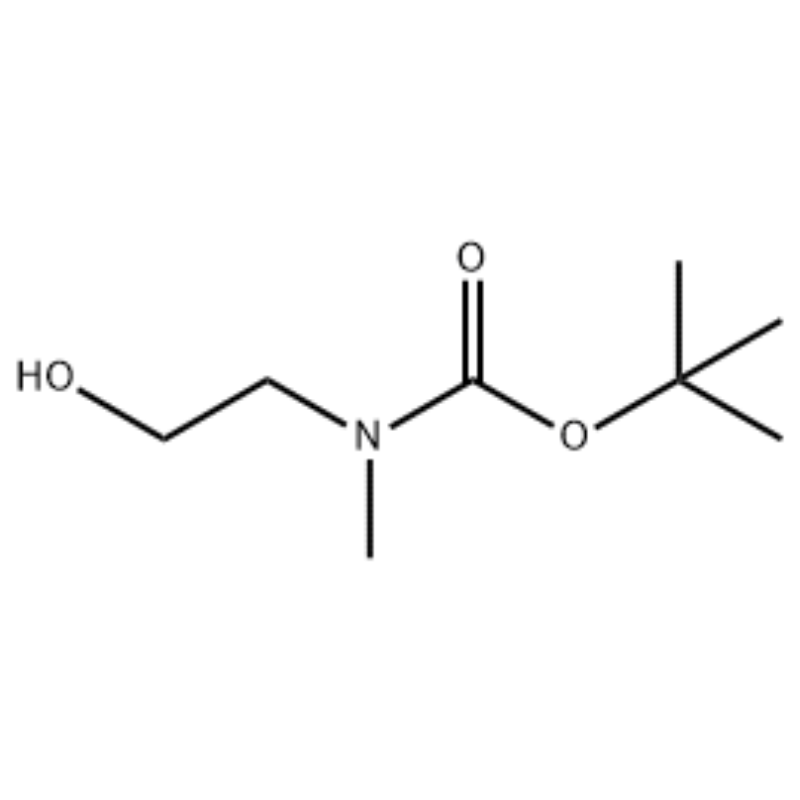
Xalka 2- (methylamino) ethanol (500 mg, 0.53 ml, 6.66 mmol) ee CH2Cl2 (20 ml) ayaa lagu daray Boc2O (1.48 g, 6.79 mmol), ka dibna walaaqaya heerkulka qolka 1 saac.Xalka falcelinta waxaa lagu soo saaray brine iyo CH2Cl2.Lakabka dabiiciga ah ee sidaas lagu helay ayaa lagu qallajiyey MgSO4 oo la sifeeyay.Kadibna, shaandheynta ayaa lagu uruuriyay vacuo si loo helo isku-dhafka shayga (saliid aan midab lahayn, tiro);1H NMR (200 MHz, CDCl3) delta 3.74 (q, J= 10.5, 5.2 Hz, 2H) 3.25 (t, J= 5.2 Hz, 2H) 2.91 (s, 3H) 1.45 (s, 9H);spectrum mass m/e (xoogga qaraabada) 144 (20) 102 (24) 57 (70) 44 (100).
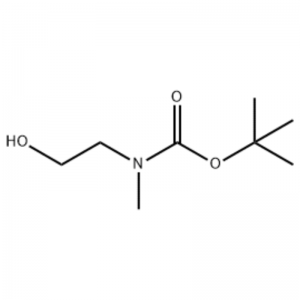
Tusaale 38;N1- (3-Fluoro-4- (2- (1- (2- (methylamino)) ethyl)) -1H-imidazol-4-yl) thieno [3,2-b] pyridin-7-yloxy) phenyl) -N3 -(2-methoxyphenyl) malonamide (96);Tallaabada 1: tert-Butyl 2-hydroxyethyl (methyl) karbamate (97) (J. Med. Chem., 1999, 42, 11, 2008) Si xalalka 2- (methylamino) ethanol (5.0 g, 67 mmol) gudaha THF (50 ml) at RT waxaa lagu daray Boc2O (15.7 g, 72 mmol) iyo isku darka falcelinta waxaa lagu walaaqay RT 4 saacadood.Isku darka falcelinta ayaa lagu ururiyey qallayl iyo xarunta cinwaanka 97 si toos ah ayaa loo isticmaalay tallaabada xigta iyada oo aan lahayn nadiifin dheeraad ah (11.74 g, 100% dhalidda).MS (m/z): 176.2 (M+H).
Diyaarinta l-2-[4-Bromo-2-- (4-oxo-2-ftiotaioxo1hiazolidin-5-ylidenemefliyl) phenoxy] efliyl-3-efliyl-l- methylurea (Compoiotamd 161) Tallaabada 1: Synthesis of t-butyl2- hydroxyethylmethylcarbamate;Xalka 2- (methylamino) ethanol (500 mg, 0.53 ml, 6.66 mmol) ee CH2Cl2 (20 ml) ayaa lagu daray BoC2O (1.48 g,6.79 mmol), ka dibna walaaqaya heerkulka qolka 1 saac.Xalka falcelinta waxaa lagu soo saaray brine iyo CH2Cl2.Lakabka dabiiciga ah ee sidaas lagu helay ayaa lagu qallajiyey MgSO4 oo la sifeeyay.Kadib, shaandheynta waxaa lagu uruuriyay vacuo si loo helo iskudarka shayga (saliid aan midab lahayn, tiro); 1HNMR (200 MHz, CDCl3) delta 3.74 (q, J= 10.5, 5.2 Hz, 2H) 3.25 (t, J= 5.2 Hz, 2H) 2.91 (s, 3H) 1.45 (s, 9H);spectrum mass m/e (xoogga qaraabada) 144 (20) 102 (24) 57 (70) 44 (100).
2- (methylamino) ethanol (90.1 g, 1.2 mol) ayaa lagu milmay 1.2 L of methylene chloride, iyo BoC2O (218 g, 1 mol) ayaa si tartiib ah loogu daray iyada oo la walaaqayo 00C, oo ay ku xigto heerkulka qolka 3 saacadood.Isku darka falcelinta ayaa si isdaba joog ah loogu dhaqay 700 ml oo ah xal ammonium chloride ah oo buuxa, iyo 300 ml oo biyo ah.Isku darka la dhaqay ayaa fuuqbaxay iyadoo la isticmaalayo sodium sulfate anhydrous waxaana lagu uruuriyay cadaadis la dhimay, si loo helo xarunta (a) (175 g, 1 mol, 100%) sida saliid aan midab lahayn.TLC: Rf = 0.5 (50% EtOAc in Hex) lagu sawiray Ce-Mo stain1H NMR (600MHz, CDCl3) delta 1.47 (s, 9H), 2.88 (br s, IH), 3.41 (br s, 2H), 3.76 (br s, 2H).
90.1 g (1.2 mol) ee 2- (methylamino) ethanol ayaa lagu milmay 1.2 L of methylene chloride, 218 g (1 mol) ee Boc2O ayaa si tartiib tartiib ah loogu daray halka xalka natiijada lagu walaaqay 0C, iyo xalalka ka soo baxay ayaa lagu kiciyay heerkulka qolka 3 saacadood.Isku darka falcelinta ayaa si isdaba joog ah loogu dhaqay 700 mL oo ah xal ammonium chloride ah oo buuxa iyo 300 mL oo biyo ah, fuuqbaxa iyadoo la isticmaalayo sodium sulfate anhydrous, ka dibna waxaa la saaray cadaadis la dhimay si loo helo 175 g (1 mol) oo ah iskudar saliid achromic ah oo ay ilaaliso Kooxda Boc (wax-soo-saarka: 100%).[0140] 1H NMR (600MHz, CDCl3) delta 7.84 (brs, 2H), 7.76 (br s, 2H), 4.34 (d, J = 15.0 Hz, 2H), 3.63 (br s, 2H), 3.04 (d) , J = 15.0 Hz, 3H), 1.46 (d, J = 16.2 Hz, 9H) [0141] 90 g (0.514 mol) ee xarunta la helay ayaa lagu milmay 1.5 L ee tetrahydrofuran, 88.0 g (539 mol) ee N- hydroxyphthalimide iyo 141 g (0.539 mol) oo ah triphenylphosphine ayaa lagu daray, 106 mL (0.539 mol) ee diisopropyl azodicarboxylate ayaa si tartiib ah loogu daray iyada oo la walaaqayo xalka ka soo baxay 0C, xalka ka soo baxayna waxaa lagu walaaqay 3 saacadood halka heerkulku uu jiray heerkulka qolka.Ka dib marka la isku daro falcelinta falcelinta cadaadiska hoos u dhaca, 600 mL oo isopropylether ah ayaa lagu daray, xalka natiijada waxaa lagu walaaqay 0C 1 saac, iyo nooca adag ee triphenylphosphine oxide ayaa la sifeeyay.Maaddada adag waxaa lagu dhaqay 200 mL oo isopropylether ah oo lagu qaboojiyey 0C waxaana lagu soo ururiyay shaandhada ugu horreysa, natiijada ka soo baxdana waxaa lagu uruuriyay cadaadis la dhimay si loo helo 198 g oo ah isku dhafka Compound XX iyo diisopropyl hydrazodicarboxylate ee saamiga isku dhafka ah ee 10 ilaa 15%. (wax soo saar: 120%).[0142] 1H NMR (600MHz, CDCl3) delta 7.84 (br s, 2H), 7.76 (br s, 2H), 4.34 (d, J = 15.0 Hz, 2H), 3.63 (br s, 2H), 3.04 (d) , J = 15.0 Hz, 3H), 1.46 (d, J= 16.2 Hz, 9H)
 Dhismaha 12, No.309, South 2nd Road, Aagga Horumarinta Dhaqaalaha, Degmada Longquanyi, Chengdu, Sichuan, Shiinaha.
Dhismaha 12, No.309, South 2nd Road, Aagga Horumarinta Dhaqaalaha, Degmada Longquanyi, Chengdu, Sichuan, Shiinaha. amy@enlaibio.com / cynthia@enlaibio.com / edison@enlaibio.com / daisy@enlaibio.com
amy@enlaibio.com / cynthia@enlaibio.com / edison@enlaibio.com / daisy@enlaibio.com +86 (028) 84841969
+86 (028) 84841969 +86 135 5885 5404
+86 135 5885 5404



















.png)


